Hawaii’s COVID-19 Vaccination Plan; Who, Where, When
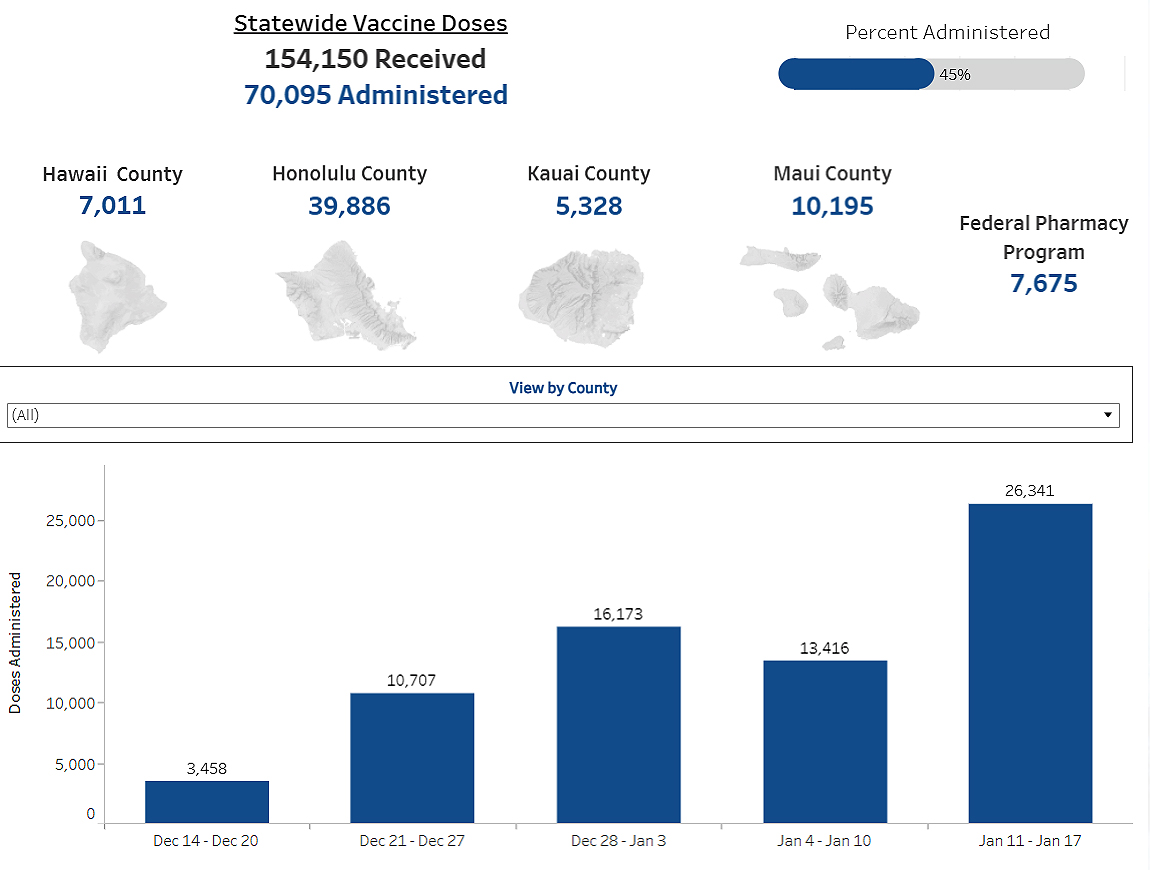
Locally, more than 80% of the COVID-19 related deaths in Hawaii have been among people older than 65. So far, approximately 35,000 doses have been administered to mostly medical workers, first responders and individuals 75 and older across the islands, according to the Hawaii Department of Health.
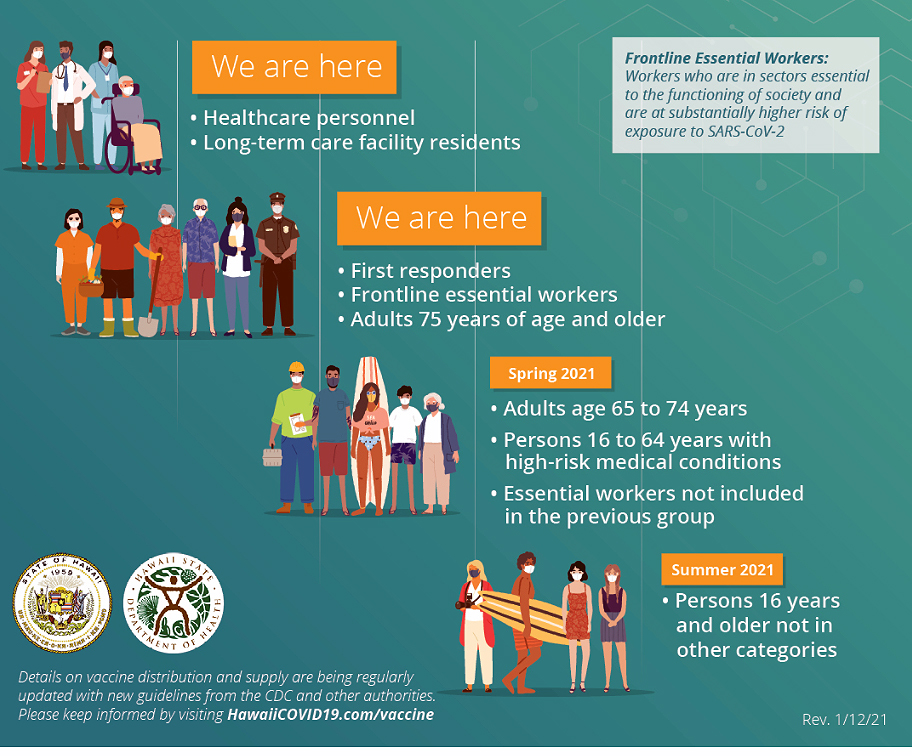
Stage One, per CDC vaccination guidelines, call for vaccine availability to be divided into three layers of availability. Previously reported by Hawaii’s DOH, the state’s COVID-19 Vaccination Plan foresees 883,600 people in Hawaii will be vaccinated during the first three layers in Stage one. Thereafter, in Stage two, anyone left unvaccinated will have access to the vaccine.
- Stage 1.a – will be divided into two phases, first it covers high-risk health workers and first-responders followed by people with comorbidities and underlying health conditions that put them at high risk and adults over age 65 living in “overcrowded settings.” It’s estimated 121,000 will be vaccinated during stage one.
- Stage 1.b – includes K-12 teachers and school staff; critical risk workers; people with comorbidities and underlying health conditions that put them at moderately high risk; people in homeless shelters or group homes; incarcerated individuals and staff at incarceration facilities; and all adults over age 65 (with or without pre-existing medial conditions). An estimated 450,000 people would be vaccinated during stage two.
- Stage 1.c – an additional 403,000 people would be vaccinated, including young adults between age 18 and 24 and children up to age 17 and workers in industries and occupations not included in earlier stages.
- Stage Two – will focus on vaccinating an undetermined number of Hawaii residents who did not have access to or receive a vaccination during the earlier stages.
State Vaccination Plan Ignores “Pre-existing Conditions”
Hawaii residents between 65 and 75, with pre-existing conditions, will likely be forced to wait until possible Spring of 2021 (stage 1.b plan timeline projection) before they have access to the vaccine.
This group of residents (65-74) has a greater chance for medical complications and death from contracting COVID-19 than those individuals without pre-existing conditions. Yet, they are excluded from the 1.a priority group before becoming eligible to receive the life-saving COVID-19 vaccination.
To date, of the COVID-19 identified in Hawaii, 7% have required hospitalization, 347 have died, with a total statewide case count of 323,443, (93% are residents).
So far, nearly 71,000 doses have been administered, representing about 5% of the state’s population. Statewide vaccinations to date have been limited to medical workers, first responders, individuals 75 and older, and so-called essential workers, according to the Hawaii Department of Health.
As of January 24, 2021
For Hawaii Island, Queen’s North Hawaii Community Hospital in Waimea reported that some of the eight trays of 975 doses were shipped earlier to the neighboring islands. Confirmed vaccination distribution centers include Kona Community Hospital, HHSC’s West Hawaii Region, which includes Kohala Hospital, Alii Health Center and the Kona Ambulatory Surgery Center. Kaiser Permanente is also vaccinating pre-qualified recipients in Oahu, but did not respond to our request for information on the health system’s vaccination plans for Hawaii Island. Federal guidelines previously called for major pharmacies participation in the distribution of vaccine.
On the new president’s first day, President Biden, invoking his emergency presidential powers enacted the “Defense Production Act”. The stated goal is making available to all fifty states enough vaccines to inoculate at least 100 million Americans within his first 100 days.
Hawaii’s Vaccination Plan Slowly Rolls Out, Governor Ige tells public to be patient
States struggle to decide who should get Covid vaccine first
The federal government’s vaccine, therapeutics and medical supply development initiative, Operation Warp Speed, has spent more than $18bn to get pharmaceutical interventions for Covid-19 to market. But just a fraction of that has been allocated for distribution in the most logistically complex vaccination campaign in American history.
A widely cited study in the American Journal of Preventive Medicine found roughly 75% of US residents would need to develop immunity to “extinguish the epidemic”. That level has never been reached by the annual flu vaccine, which usually around half the population takes. The vaccines are untested in children, which means 70 million Americans under age 16 will not be eligible to receive the vaccine, underscoring the need for high adult uptake.
Two vaccine candidates lead the race. One, Pfizer and BioNTech, which has an infamously difficult -94F (-70C) ultra-cold storage requirement and was just approved by the United Kingdom. A second is developed by Moderna and the National Institute of Allergy and Infectious Diseases. If independent analyses bear out publicly released data, both would be among the most effective vaccines in medicine.
With both vaccines now approved for distribution, federal authorities believe there will be enough doses initially to vaccinate 20 million people. That may still fall short of vaccinating everyone in the highest-priority groups, and a drop in bucket in a country with a population of 331 million citizens.
There are roughly 20 million healthcare workers in the US, ranging from nurses to home health aides to hospital housekeepers. Another 3 million people live in long-term care facilities, such as nursing homes and assisted living facilities.
What the polls say about Americans’ willingness to get the vaccine
Five pollsters that have recently tracked how Americans feel about a coronavirus vaccine have found a mixed willingness to receive one, with a range of 45 to 61 percent of the public saying they will or are likely to get the injections.
The seven surveys, conducted by five firms since Nov. 1, illustrate the possible challenges that may await public health officials as they seek to inoculate Americans against a virus that has sickened more than 16 million and killed over 300,000.
Pfizer and Moderna, the companies behind the two vaccine front-runners, have said their drugs are safe and effective. But many of those who are unwilling or unsure about coronavirus vaccination say they are not very confident in the safety of the development and approval process or in the federal government’s ability to oversee it, the polls found.
In a Quinnipiac University poll published Wednesday, 6 in 10 registered voters said they’re willing to get a vaccine “if it is approved by government health officials.” But 37 percent said they would take the vaccine as soon as it’s available to them, while 41 percent said they would “wait a few months.”


Leave a Reply
Join the Community discussion now - your email address will not be published, remains secure and confidential. Mahalo.